| All rights reserved. |
| Step 5 |
Our basic explanation is split into several steps

Page 1
Page 2
More information on how it works
VPPEM is unique, it images very low energy photoelectrons.
VPPEM can use very low secondary electrons for Partial-Yield NEXAFS.
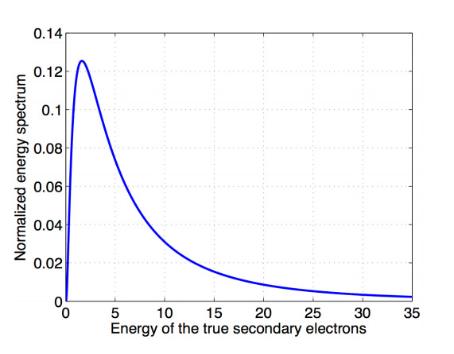
The low energy part of the spectrum is shown on the right. This is the true
secondary electron spectrum and is a large signal making up approximately
10% of the total secondary electron signal.
The VPPEM energy window is in the range of 100 mV, and the window can be
scanned across the secondary electron spectrum from zero eV up to several
hundred eV.
What are the advantages of using the low energy signal?
1. The best spatial resolution for VPPEM is at low energies, see Step 7.
2. The mean free path, or the sampling depth changes rapidly at low
energies, and a change in sampling energy can be used for depth profiling.
3. It is a strong signal.
4. No other technique can use it as accurately as VPPEM, so we have
a new tool.
The low energy part of the spectrum is shown on the right. This is the true
secondary electron spectrum and is a large signal making up approximately
10% of the total secondary electron signal.
The VPPEM energy window is in the range of 100 mV, and the window can be
scanned across the secondary electron spectrum from zero eV up to several
hundred eV.
What are the advantages of using the low energy signal?
1. The best spatial resolution for VPPEM is at low energies, see Step 7.
2. The mean free path, or the sampling depth changes rapidly at low
energies, and a change in sampling energy can be used for depth profiling.
3. It is a strong signal.
4. No other technique can use it as accurately as VPPEM, so we have
a new tool.
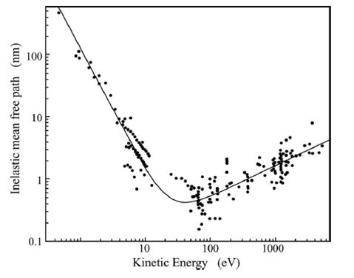
Mean free path for low energy electrons, from Seah and Dench

The VPPEM signal consists of the absorption yield signal, and the
photoemission peak closely mixed together.
This figure illustrates these sources of the signal in a schematic photoelectron
emission energy level diagram.
This illustration is particular for VPPEM which uses a biased sample to set the
energy of the detected electrons, but is also relevant to the interpretation of
PEEM data at very low energies where the analyzing energy is scanned and very
similar results are obtained.
In the figure the vertical direction is energy, and the horizontal direction
represents distance out of the sample surface. This illustrates the case of an
aluminum sample with an oxidized surface. The metal is on the left, and the
VPPEM microscope is on the right.
The sample is irradiated by x-ray photons, and if a photon, ħω, has sufficient
energy it can create a core hole in the metal by removing an electron in a core
state to an empty state above the Fermi level.
In the case of an insulating oxide, the core electron is removed to the conduction
band edge (CBE). We expect, in theory, the Fermi level in the oxide to be midway
between the conduction band edge, and the top of the valence band (VBE).
The photoelectron transitions in the illustration are indicated by the blue vertical
arrows beginning with a circle to represent the core hole remaining. Only the
aluminum metal transitions have been shown.
photoemission peak closely mixed together.
This figure illustrates these sources of the signal in a schematic photoelectron
emission energy level diagram.
This illustration is particular for VPPEM which uses a biased sample to set the
energy of the detected electrons, but is also relevant to the interpretation of
PEEM data at very low energies where the analyzing energy is scanned and very
similar results are obtained.
In the figure the vertical direction is energy, and the horizontal direction
represents distance out of the sample surface. This illustrates the case of an
aluminum sample with an oxidized surface. The metal is on the left, and the
VPPEM microscope is on the right.
The sample is irradiated by x-ray photons, and if a photon, ħω, has sufficient
energy it can create a core hole in the metal by removing an electron in a core
state to an empty state above the Fermi level.
In the case of an insulating oxide, the core electron is removed to the conduction
band edge (CBE). We expect, in theory, the Fermi level in the oxide to be midway
between the conduction band edge, and the top of the valence band (VBE).
The photoelectron transitions in the illustration are indicated by the blue vertical
arrows beginning with a circle to represent the core hole remaining. Only the
aluminum metal transitions have been shown.
We can accelerate electrons in the magnet field by biasing the sample
negatively. The magnetic field is so strong that there is no distortion of the
image due to electrostatic fringing fields around the sample.
Even for very low energy electrons < 1.0 eV energies, the electron trajectories
are unchanged when they are accelerated.
We take advantage of this by fixing the energy of the electrons going through
the electrostatic lenses and CHA, and changing the sample bias.
We accelerate all electrons up to 50 eV, and this means we can easily image
even the lowest energy electrons emitted from the sample without changing
the magnification of the electron optical system.
negatively. The magnetic field is so strong that there is no distortion of the
image due to electrostatic fringing fields around the sample.
Even for very low energy electrons < 1.0 eV energies, the electron trajectories
are unchanged when they are accelerated.
We take advantage of this by fixing the energy of the electrons going through
the electrostatic lenses and CHA, and changing the sample bias.
We accelerate all electrons up to 50 eV, and this means we can easily image
even the lowest energy electrons emitted from the sample without changing
the magnification of the electron optical system.

VPPEM looks at two features that are in the low energy signal, the directly
emitted low energy photoelectron, and the change in signal as the photon
energy sweeps across a core energy level.
The directly emitted low energy photoelectron is from the an x-ray photon
ejecting a core electron from an atom in the sample. Because we can look at
low energy electrons, and change the energy we can use this to change the
sampling depth and do depth profiles.
The low energy true secondary electrons are created by a multistage
process that starts with the core ionization.
First, an x-ray photon ejects a core electron from an atom in the sample.
Second, an Auger electron is emitted from the photo-excited atom.
Third, the Auger electrons lose energy, largely through bulk plasmon
excitation.
Fourth, low energy electrons which by subsequent losses emerge as a
cascade of low energy electrons into the vacuum. This is the signal we use
for Partial Yield NEXAFS.
emitted low energy photoelectron, and the change in signal as the photon
energy sweeps across a core energy level.
The directly emitted low energy photoelectron is from the an x-ray photon
ejecting a core electron from an atom in the sample. Because we can look at
low energy electrons, and change the energy we can use this to change the
sampling depth and do depth profiles.
The low energy true secondary electrons are created by a multistage
process that starts with the core ionization.
First, an x-ray photon ejects a core electron from an atom in the sample.
Second, an Auger electron is emitted from the photo-excited atom.
Third, the Auger electrons lose energy, largely through bulk plasmon
excitation.
Fourth, low energy electrons which by subsequent losses emerge as a
cascade of low energy electrons into the vacuum. This is the signal we use
for Partial Yield NEXAFS.
After the initial photoelectron event, the remaining core hole is filled with an electron
creates a flux of inelastically scattered electrons over a wide energy range. These
electrons form the secondary electron distribution when they leave the sample.
The increase in the number of secondary electrons as the photon energy is swept from
a low energy up across the core absorption energy is detected by the spectrometer as a
Partial Yield NEXAFS signal with a prominent feature at the core edge absorption
energy.
As the photon energy is raised to several eV above the core absorption energy, the
directly excited photoelectron, the XPS electron, ejected from the aluminum metal core
hole can escape the surface directly. The directly emitted electron must have a kinetic
energy in the solid that is greater than the work function. An aluminum metal
photoelectron also has to travel through the oxide over layer without being absorbed.
If the potential bias of the sample has been set so that the energy of the directly excited
electron in the vacuum coincides with the energy window of the spectrometer, then the
electron is detected.
If the sample bias is fixed, and the photon energy is increased, the electron energy will
move through the spectrometer window to produce a peak in the spectrometer signal,
the photo peak.
The photo peak will be superimposed on the higher energy part of the Partial Yield
NEXAFS structure because the core edge and the photo peak are only a few eV apart.
See the next page.
creates a flux of inelastically scattered electrons over a wide energy range. These
electrons form the secondary electron distribution when they leave the sample.
The increase in the number of secondary electrons as the photon energy is swept from
a low energy up across the core absorption energy is detected by the spectrometer as a
Partial Yield NEXAFS signal with a prominent feature at the core edge absorption
energy.
As the photon energy is raised to several eV above the core absorption energy, the
directly excited photoelectron, the XPS electron, ejected from the aluminum metal core
hole can escape the surface directly. The directly emitted electron must have a kinetic
energy in the solid that is greater than the work function. An aluminum metal
photoelectron also has to travel through the oxide over layer without being absorbed.
If the potential bias of the sample has been set so that the energy of the directly excited
electron in the vacuum coincides with the energy window of the spectrometer, then the
electron is detected.
If the sample bias is fixed, and the photon energy is increased, the electron energy will
move through the spectrometer window to produce a peak in the spectrometer signal,
the photo peak.
The photo peak will be superimposed on the higher energy part of the Partial Yield
NEXAFS structure because the core edge and the photo peak are only a few eV apart.
See the next page.
This figure shows a VPPEM spectrum. The spectrum is along the
photon energy axis for a fixed detection energy of 1.0 eV. This data is
from an oxidized AL surface taken from a spectral image series with
photon energies from 72.5 eV to 85.0 eV in 0.5 eV steps.
The NEXAFS signal is at 74.5 eV, and the Al metal XPS signal is at the
work function plus the detected electron energy, 1.0 eV, higher in
energy.
Note this type of spectrum is unique to VPPEM, and is not either a
typical NEXAFS or an XPS spectrum.
photon energy axis for a fixed detection energy of 1.0 eV. This data is
from an oxidized AL surface taken from a spectral image series with
photon energies from 72.5 eV to 85.0 eV in 0.5 eV steps.
The NEXAFS signal is at 74.5 eV, and the Al metal XPS signal is at the
work function plus the detected electron energy, 1.0 eV, higher in
energy.
Note this type of spectrum is unique to VPPEM, and is not either a
typical NEXAFS or an XPS spectrum.

| How it works |